N-Heterocyclic carbene-catalyzed stereoselective construction of olefinic carbon–sulfur bonds via cross-coupling reaction of gem-difluoroalkenes and thiols†
Abstract
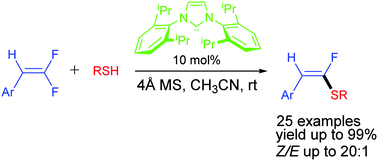
A novel organocatalytic olefinic carbon–sulfur bond forming reaction was developed. Under the catalysis of 10 mol% stable N-heterocyclic carbene, thiols undergo direct nucleophilic substitution reaction with gem-difluoroalkenes to produce α-fluorovinyl thioethers in high yields with excellent Z-selectivity. In this process, bases are not necessary.


 Please wait while we load your content...
Please wait while we load your content...