Amidation of unactivated ester derivatives mediated by trifluoroethanol†
Abstract
A catalytic amidation protocol mediated by 2,2,2-trifluoroethanol has been developed, facilitating the condensation of unactivated esters and amines, furnishing both secondary and tertiary amides. The complete scope and limitations of the method are described, along with modified conditions for challenging substrates such as acyclic secondary amines and chiral esters with retention of chiral integrity.
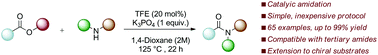


 Please wait while we load your content...
Please wait while we load your content...