A method for accessing sulfanylfurans from homopropargylic alcohols and sulfonyl hydrazides†
Abstract
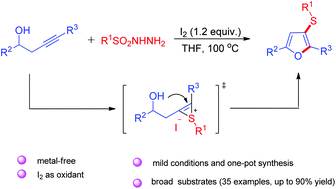
A sulfenylation/cyclization reaction has been developed for the synthesis of sulfanylfurans in the presence of iodine. These processes provide facile strategies to construct C–S/O bonds, and expand the methods of synthesizing sulfanylfurans. In this transformation, various groups on homopropargylic alcohols and sulfonyl hydrazides react smoothly and the desired sulfanylfurans are obtained in moderate to good yields.


 Please wait while we load your content...
Please wait while we load your content...