Iodine-catalyzed sulfenylation of pyrazolones using dimethyl sulfoxide as an oxidant†
Abstract
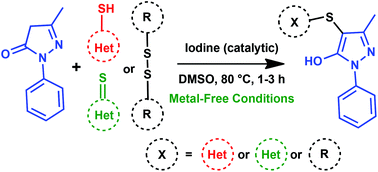
An iodine catalyzed sulfenylation of pyrazolones with a diverse range of heterocyclic thiols, heterocyclic thiones and disulfides has been described using dimethyl sulfoxide as an oxidant, which is an inexpensive, readily available and green oxidant. The present methodology exhibits a wide range of substrate scope and targeted products were obtained in good to excellent yields under metal-free conditions in a short duration. This methodology provides a simple process for the formation of C–S bonds through the thioetherification of pyrazolones.


 Please wait while we load your content...
Please wait while we load your content...