Direct palladium-mediated on-resin disulfide formation from Allocam protected peptides†
Abstract
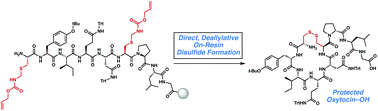
The synthesis of disulfide-containing polypeptides represents a long-standing challenge in peptide chemistry, and broadly applicable methods for the construction of disulfides are in constant demand. Few strategies exist for on-resin formation of disulfides directly from their protected counterparts. We present herein a novel strategy for the on-resin construction of disulfides directly from Allocam-protected cysteines. Our palladium-mediated approach is mild and uses readily available reagents, requiring no special equipment. No reduced peptide intermediates or S-allylated products are observed, and no residual palladium can be detected in the final products. The utility of this method is demonstrated through the synthesis of the C-carboxy analog of oxytocin.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...