Synthesis of 2,3-dihydro-1H-pyrroles by intramolecular cyclization of N-(3-butynyl)-sulfonamides†
Abstract
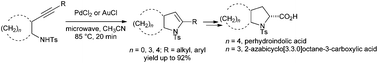
Hydroamination of 3-butynamine derivatives to give non-aromatic 2,3-dihydropyrroles was achieved by using PdCl2 or AuCl as the catalyst. With microwave-assisted heating, up to 92% isolated yield was obtained from this intramolecular 5-endo-dig cyclisation. The cyclopentane- and cyclohexane-fused 2,3-dihydropyrroles were transformed into the corresponding N-tosyl-pyrrolidine-2-carboxylic acids.


 Please wait while we load your content...
Please wait while we load your content...