A threonine turnstile defines a dynamic amphiphilic binding motif in the AAA ATPase p97 allosteric binding site†
Abstract
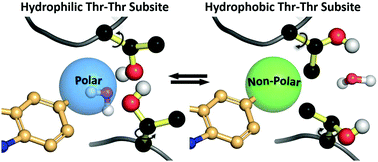
The turnstile motion of two neighboring threonines sets up a dynamic side chain interplay that can accommodate both polar and apolar ligands in a small molecule allosteric protein binding site. A computational model based on SAR data and both X-ray and cryo-EM structures of the AAA ATPase p97 was used to analyze the effects of paired threonines at the inhibitor site. Specifically, the Thr side chain hydroxyl groups form a hydrogen bonding network that readily accommodates small, highly polar ligand substituents. Conversely, diametric rotation of the χ1 torsion by 150–180° orients the side chain β-methyl groups into the binding cleft, creating a hydrophobic pocket that can accommodate small, apolar substituents. This motif was found to be critical for rationalizing the affinities of a structurally focused set of inhibitors of p97 covering a > 2000-fold variation in potencies, with a preference for either small-highly polar or small-apolar groups. The threonine turnstile motif was further validated by a PDB search that identified analogous binding modes in ligand interactions in PKB, as well as by an analysis of NMR structures demonstrating additional gear-like interactions between adjacent Thr pairs. Combined, these data suggest that the threonine turnstile motif may be a general feature of interest in protein binding pockets.


 Please wait while we load your content...
Please wait while we load your content...