cis or trans with class II diterpene cyclases†
Abstract
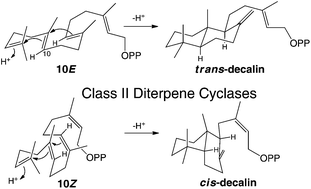
Isoprenoid precursors readily undergo (poly)cyclization in electrophilic reaction cascades, presumably as internal addition of the carbon–carbon double-bonds from neighboring isoprenyl repeats readily forms relatively stable cyclohexyl tertiary carbocation intermediates. This hypothesis is agnostic regarding alkene configuration (i.e., Z or E). Consistent with this, here it is shown that certain class II diterpene cyclases, which normally convert (E,E,E)-geranylgeranyl diphosphate to 13E-trans-decalin bicycles, will also act upon (Z,Z,Z)-nerylneryl diphosphate, producing novel 13Z-cis-decalin bicycles instead.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...