PhI(OAc)2-mediated 1,2-aminohalogenation of alkynes: a general access to (E)-4-(halomethylene)oxazolidin-2-ones†
Abstract
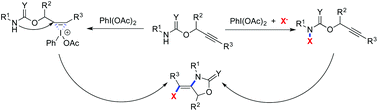
A new PhI(OAc)2-mediated 1,2-aminohalogenation of prop-2-yn-1-yl carbamates and various halogen sources is presented with excellent selectivity and high step-economy. The reaction is general and rapid for the construction of diverse (E)-4-(halomethylene)oxazolidin-2-ones through the generation of the three-membered ring or N-radical followed by intramolecular cyclization.


 Please wait while we load your content...
Please wait while we load your content...