Aerobic catalytic systems inspired by copper amine oxidases: recent developments and synthetic applications
Abstract
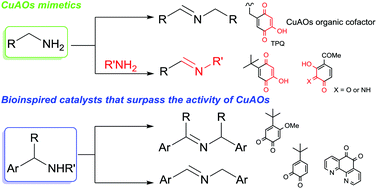
Recent efforts to design synthetic quinone-based catalysts for the efficient aerobic oxidation of amines to imines have been inspired by copper amine oxidases (CuAOs), a family of metalloenzymes which selectively converts primary amines into aldehydes, using molecular oxygen through the cooperation of a quinone-based cofactor, 2,4,5-trihydroxyphenylalanine quinone (TPQ) and a copper ion. Two distinct classes of bioinspired quinone-based catalytic systems have been developed. The first class consists of catalytic systems, which mimic the activity of CuAOs by exhibiting exquisite selectivity for primary amines. The second class consists of catalytic systems, which allow the expansion of the substrate scope to the oxidation of α-branched primary amines and secondary amines including nitrogen heterocycles, two reaction types that natural CuAOs are not able to accomplish. These catalytic oxidative green processes can be applied to the C–H functionalization of primary amines and to the synthesis of several nitrogen-containing heterocycles.
- This article is part of the themed collection: Biocatalysis: Natural and biologically inspired synthetic enzymes


 Please wait while we load your content...
Please wait while we load your content...