Intramolecular cascade rearrangements of enynamine derived ketenimines: access to acyclic and cyclic amidines†
Abstract
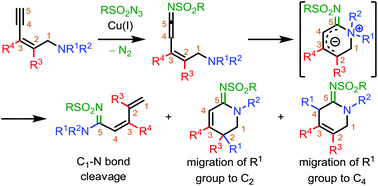
Copper-catalyzed reaction of enynamines with sulfonylazides provides acyclic and cyclic amidines. Nucleophilic addition of the tethered amino group on the in situ generated ketenimine forms a six-membered cyclic zwitterionic intermediate which facilitates migration of the tethered amino group to the C5-center giving the acyclic amidine. On the other hand, migration of a substituent on the amino group to C2- and C4-centers results in the formation of cyclic amidines. Computational studies were carried out to validate the mechanism which indicates that the product distribution of the process depends on the substitutions on the enynamine backbone.


 Please wait while we load your content...
Please wait while we load your content...