Base-mediated insertion reaction of alkynes into carbon–carbon σ-bonds of ethanones: synthesis of hydroxydienone and chromone derivatives†
Abstract
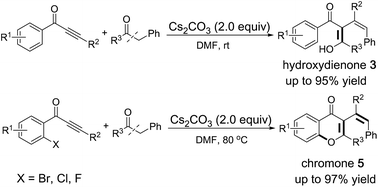
Transition-metal free insertions of alkynes into carbon–carbon σ-bonds of ethanones have been reported. These tandem reactions offer an efficient synthesis of hydroxydienones and multi-substituted chromones. This is the first example of base-promoted insertion reactions of isolated carbon–carbon triple bonds into carbon–carbon σ-bonds with active methylene compounds bearing only one electron-withdrawing group.


 Please wait while we load your content...
Please wait while we load your content...