A dual removable activating group enabled the Povarov reaction of N-arylalanine esters: synthesis of quinoline-4-carboxylate esters†
Abstract
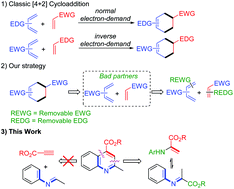
A dual removable activating group enabled Povarov reaction of N-arylalanine esters was reported. N-Arylalanine ester was utilized as the sole carbon source to generate N-arylimine and its tautomer, enamine, in situ by aerobic oxidation of sp3 C–H bonds, and then the consecutive reaction delivered the desired quinoline-4-carboxylate esters in high yields.


 Please wait while we load your content...
Please wait while we load your content...