Synthesis of chiral cyclic amines via Ir-catalyzed enantioselective hydrogenation of cyclic imines†
Abstract
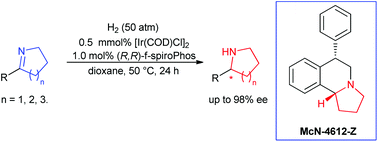
A highly enantioselective hydrogenation of cyclic imines for synthesis of chiral cyclic amines has been realized. With the complex of iridium and (R,R)-f-spiroPhos as the catalyst, a range of cyclic 2-aryl imines were smoothly hydrogenated under mild conditions without any additive to provide the corresponding chiral cyclic amines with excellent enantioselectivities of up to 98% ee. Moreover, this method could be successfully applied to the synthesis of (+)-(6S,10bR)-McN-4612-Z.


 Please wait while we load your content...
Please wait while we load your content...