Croconamides: a new dual hydrogen bond donating motif for anion recognition and organocatalysis†
Abstract
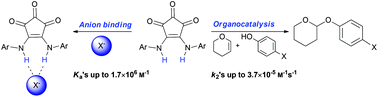
We introduce bis-aryl croconamides as a new member in the family of dual hydrogen bonding anion receptors. In this study a series of croconamides are synthesised, and the selectivity for anion binding is investigated (Cl− > Br− > I− in CH2Cl2). The croconamides exhibit different structures in the crystal phase depending on the substituents on the aromatic rings, and furthermore, the crystal structure revealed the presence of tautomers. DFT calculations elucidated the complex structures formed upon addition of anion to the croconamides, confirming the order of association constants towards the halogen anions. The use of croconamides as organocatalysts in a proof-of-concept study is demonstrated in the formation of THP ethers. In addition to this, construction of a Hammet plot further elucidates the mechanism in action on formation of THP ethers.


 Please wait while we load your content...
Please wait while we load your content...