Acid-promoted cyclization of 2,4-diaryl-1,1,1-trifluorobut-3-en-2-oles and their TMS-ethers into CF3-indenes†
Abstract
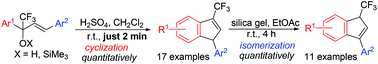
2,4-Diaryl-1,1,1-trifluorobut-3-en-2-oles and their TMS-ethers in H2SO4 at room temperature in just 2 min are quantitatively cyclized into 1-aryl-3-trifluoromethyl-1H-indenes. The reaction proceeds through an intermediate formation of the corresponding CF3-allyl cations, which are cyclized regioselectively at the allyl carbon atom most remote from the CF3-group. The obtained CF3-indenes in solution of EtOAc in the presence of silica gel at room temperature over 4 h are quantitatively isomerized into 3-aryl-1-trifluoromethyl-1H-indenes.


 Please wait while we load your content...
Please wait while we load your content...