Synthesis of oxazolidinones: rhodium-catalyzed C–H amination of N-mesyloxycarbamates†
Abstract
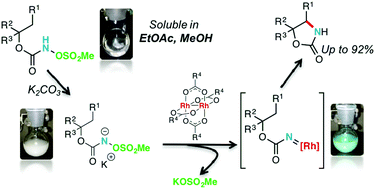
N-Mesyloxycarbamates undergo intramolecular C–H amination reactions to afford oxazolidinones in good to excellent yields in the presence of rhodium(II) carboxylate catalysts. The reaction is performed under green conditions and potassium carbonate is used, forming biodegradable potassium mesylate as a reaction by-product. This method enables the production of electron-rich, electron-deficient, aromatic and heteroaromatic oxazolidinones in good to excellent yields. Conformationally restricted cyclic secondary N-mesyloxycarbamates furnish cis-oxazolidinones in high yields and selectivity; DFT calculations are provided to account for the observed selectivity. trans-Oxazolidinones were prepared from acyclic secondary N-mesyloxycarbamates using Rh2(oct)4. The selectivity was reverted with a cytoxazone N-mesyloxycarbamate precursor using large chiral rhodium(II) carboxylate complexes, affording the corresponding cis-oxazolidinone. This orthogonal selectivity was used to achieve the formal synthesis of (−)-cytoxazone.
- This article is part of the themed collections: Celebrating excellence in research: women of organic chemistry and CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...