Synthesis of N-alkyl-3-sulfonylindoles and N-alkyl-3-sulfanylindoles by cascade annulation of 2-alkynyl-N,N-dialkylanilines†
Abstract
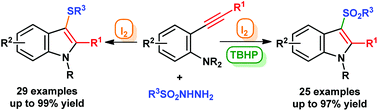
An efficient and metal-free approach to N-alkyl-3-sulfonylindoles and N-alkyl-3-sulfanylindoles from 2-alkynyl-N,N-dialkylanilines has been developed. In the presence of iodine and tert-butylhydroperoxide (TBHP), a variety of 2-alkynyl-N,N-dialkylanilines underwent a cascade radical annulation to yield 3-arylsulfonylindoles. In contrast, 3-arylsulfanylindoles were conveniently prepared by iodine mediated electrophilic annulation reactions. The present protocol uses the economical and environmentally friendly I2–TBHP or I2 system, and potentially bioactive N-alkyl-3-sulfonylindoles and N-alkyl-3-sulfanylindoles with various functional groups were successfully synthesized in moderate to good yields.
- This article is part of the themed collection: Celebrating excellence in research: women of organic chemistry


 Please wait while we load your content...
Please wait while we load your content...