Total synthesis of marine natural products separacenes A and B†
Abstract
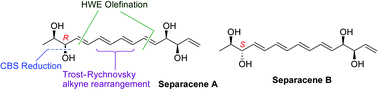
A short and convergent route for the stereoselective total synthesis of separacenes A and B has been developed using (+)-methyl D-lactate and D-(−)-tartaric acid as the chiral pools. The characteristic features of this synthesis include the Trost–Rychnovsky alkyne rearrangement to construct the C7–C9 conjugated diene, the Horner–Wadsworth–Emmons olefination to form the C5–C6 and C11–C12 olefins and the Corey–Bakshi–Shibata reaction to install the C-13 hydroxy functionality.


 Please wait while we load your content...
Please wait while we load your content...