A carbonyl reductase from Candida parapsilosis ATCC 7330: substrate selectivity and enantiospecificity†
Abstract
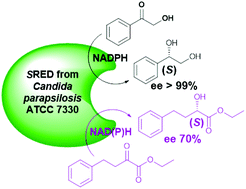
Candida parapsilosis ATCC 7330, a rich source of highly stereospecific oxidoreductases, catalyzes oxidation–reduction of a plethora of compounds yielding industrially important intermediates. An (S)-specific carbonyl reductase (SRED) purified and characterized from this yeast is reported here. (R)-Specific carbonyl reductase (CpCR) was reported by us earlier. SRED asymmetrically reduces ketones with excellent enantiospecificity (ee > 99%) and α-ketoesters with higher catalytic activity but moderate enantiospecificity (ee 70%) in the presence of NADPH. Minimal activity is shown towards the reduction of aldehydes. While the reduction of α-ketoesters with SRED can occur with either NADPH or NADH, for ketone reduction SRED requires NADPH specifically. SRED with a subunit molecular weight of 30 kDa shows optimal activity at pH 5.0 and 25 °C, and its activity is affected by Cu2+. Taken together, SRED and CpCR offer substrates which on asymmetric reduction give products of opposite absolute configurations.
- This article is part of the themed collection: Biocatalysis: Natural and biologically inspired synthetic enzymes


 Please wait while we load your content...
Please wait while we load your content...