Asymmetric synthesis of vinylogous β-amino acids and their incorporation into mixed backbone oligomers†
Abstract
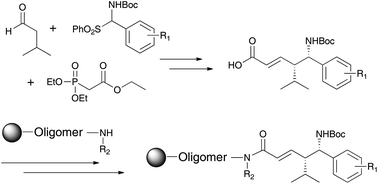
Chiral vinylogous β-amino acids (VBAA) were synthesized using enantioselective Mannich reactions of aldehydes with in situ generated N-carbamoyl imines followed by a Horner–Wadsworth–Emmons reaction. The efficiency with which these units could be incorporated into oligomers with different moieties on the C- and N-terminal sides was established, as was the feasibility of sequencing oligomers containing VBAAs by tandem mass spectrometry. The data show that VBAAs will be useful building blocks for the construction of combinatorial libraries of peptidomimetic compounds.


 Please wait while we load your content...
Please wait while we load your content...