Site-selective anion recognition of an interlocked dimer†
Abstract
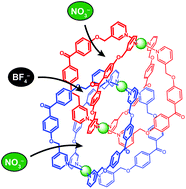
Interlocked dimer 2, which is composed of two physically interlocked monomers 1, has three cavities (cavity A × 2 and cavity B × 1) and can encapsulate three anions, such as NO3− and BF4−, one anion per cavity. There are six possible encapsulation patterns, A–F; two (A and F) contain only one kind of anion and the others (B–E) contain both NO3− and BF4− at the same time with different ratios and with different positions. Anion competition experiments showed that in addition to F, which encapsulates three NO3− ions, C, in which NO3− and BF4− ions are captured in cavities A and cavity B, respectively, was selectively formed. Detailed investigations have revealed that B–E were formed by dimerization, but three of the four were subjected to anion exchange and converged into C or F. This selective formation can be explained by the fact that NO3− is a better anion template than BF4−, as well as the molecular structure of the interlocked dimer; cavities A are surrounded by four bridging ligands and can be accessed by free anions, whereas no space available for anion exchange is present around cavity B because this cavity is surrounded by eight bridging ligands. Therefore, the BF4− ions in cavities A are expelled by free NO3−, but the BF4− ion in cavity B is not, resulting in the selection of C and F. We have found that the volume of the cavity influenced anion recognition. New interlocked dimer 3, which has smaller cavities than those of 2, captured three NO3− ions to form F, whereas only a small amount of an interlocked dimer that contains both NO3− and BF4− was formed.


 Please wait while we load your content...
Please wait while we load your content...