One-step formation of dihydrofuranoindoline cores promoted by a hypervalent iodine reagent†
Abstract
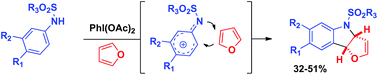
Treatment of various substituted anilines containing a sulfonyl group in the presence of a hypervalent iodine reagent, a perfluorinated alcohol and furan promotes a formal dearomatizing [2 + 3] cycloaddition process, leading to a dihydrofuranoindoline core in useful yields. Thereafter, these functionalized systems can be used for further transformations, yielding potential key intermediates for the total synthesis of complex natural products.
- This article is part of the themed collection: CSC100: Celebrating Canadian Chemistry


 Please wait while we load your content...
Please wait while we load your content...