Asymmetric organocatalytic synthesis of tertiary azomethyl alcohols: key intermediates towards azoxy compounds and α-hydroxy-β-amino esters†
Abstract
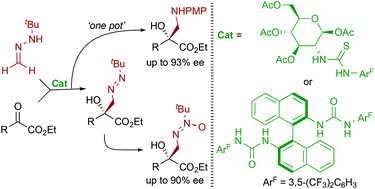
A series of peracylated glycosamine-derived thioureas have been synthesized and their behavior as bifunctional organocatalysts has been tested in the enantioselective nucleophilic addition of formaldehyde tert-butyl hydrazone to aliphatic α-keto esters for the synthesis of tertiary azomethyl alcohols. Using the 1,3,4,6-tetra-O-acetyl-2-amino-2-deoxy-β-D-glucosamine derived 3,5-bis-(trifluoromethyl)phenyl thiourea the reaction could be accomplished with high yields (75–98%) and moderate enantioselectivities (50–64% ee). Subsequent high-yielding and racemization-free tranformations of both aromatic- and aliphatic-substituted diazene products in a one pot fashion provide a direct entry to valuable azoxy compounds and α-hydroxy-β-amino esters.


 Please wait while we load your content...
Please wait while we load your content...