Efficient construction of C–N and C–S bonds in 2-iminothiazoles via cascade reaction of enaminones with potassium thiocyanate†
Abstract
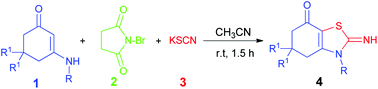
A novel and highly efficient protocol has been developed for the regioselective synthesis of 2-iminothiazole derivatives with potential biochemical interest by the reaction of enaminones, potassium thiocyanate (KSCN), and N-bromo succinimide (NBS) under mild conditions. The reaction proceeds via the formation of α-bromo enaminones as a versatile intermediate followed by thiocyanation/intramolecular cyclization in a one pot manner. The developed method is particularly attractive due to various advantages including operational simplicity, mild conditions, being catalyst free, and high bond-forming efficiency. The proposed protocol explores the synthetic routes of thiazoles by using various functional enaminones.


 Please wait while we load your content...
Please wait while we load your content...