Inter- and intramolecular aldol reactions promiscuously catalyzed by a proline-based tautomerase†
Abstract
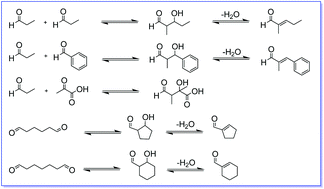
The enzyme 4-oxalocrotonate tautomerase (4-OT), which in nature catalyzes a tautomerization step as part of a catabolic pathway for aromatic hydrocarbons, was found to promiscuously catalyze different types of aldol reactions. These include the self-condensation of propanal, the cross-coupling of propanal and benzaldehyde, the cross-coupling of propanal and pyruvate, and the intramolecular cyclizations of hexanedial and heptanedial. Mutation of the catalytic amino-terminal proline (P1A) greatly reduces 4-OT's aldolase activities, whereas mutation of another active site residue (F50A) strongly enhances 4-OT's aldolase activities, indicating that aldolization is an active site process. This catalytic promiscuity of 4-OT could be exploited as starting point to create tailor-made, artificial aldolases for challenging self- and cross-aldolizations.
- This article is part of the themed collection: Biocatalysis: Natural and biologically inspired synthetic enzymes


 Please wait while we load your content...
Please wait while we load your content...