Phenacyl azides as efficient intermediates: one-pot synthesis of pyrrolidines and imidazoles†
Abstract
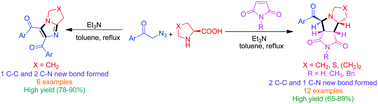
Phenacyl azides were decomposed in basic media to generate N-unsubstituted imines which were reacted with cyclic amino acids to give an azomethine ylide that underwent [3 + 2] cycloaddition with maleimides and N-unsubstituted imines to yield various diastereoselective pyrrolidines and imidazoles respectively in a one-pot three component manner with good to excellent yields.


 Please wait while we load your content...
Please wait while we load your content...