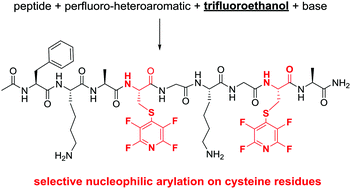
Abstract
The SNAr arylation of peptides with perfluoroaromatics provides a route by which to install a useful chemical handle that enables both 19F-NMR analysis and further chemical modification. However, chemo-selective arylation in peptides containing multiple nucleophilic side chains currently presents a challenge to the field. Herein, we demonstrate that employing 2,2,2-trifluoroethanol (TFE) as a solvent in peptide SNAr reactions significantly improves nucleophile-selectivity when compared to N,N′-dimethylformamide (DMF).


 Please wait while we load your content...
Please wait while we load your content...