Rapidly accessible “click” rotaxanes utilizing a single amide hydrogen bond templating motif†
Abstract
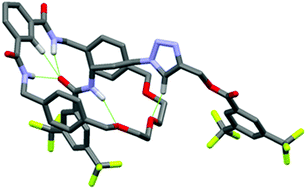
The synthesis of hydrogen bond templated rotaxanes using the CuAAC click reaction has been achieved in yields of up to 47%, employing near stoichiometric equivalents of macrocycle and readily prepared azide and alkyne half-axle components. Interlocked structure formation has been confirmed by NMR spectroscopy and mass spectrometry. Density functional theory calculations support 1H NMR spectroscopic analysis that the macrocycle resides over the amide of the axle component, rather than the newly formed triazole, as a result of more favourable hydrogen bond interactions.


 Please wait while we load your content...
Please wait while we load your content...