Thiyl radical-mediated cyclization of ω-alkynyl O-tert-butyldiphenylsilyloximes†
Abstract
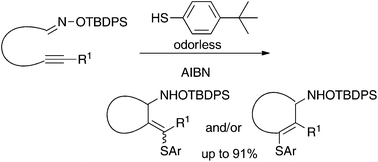
ω-Alkynyl O-tert-butyldiphenylsilyloximes, upon treatment with odorless 4-tert-butylbenzenethiol in the presence of azobisisobutyronitrile (AIBN) in refluxing benzene, underwent addition of a thiyl radical to the alkynyl group followed by radical cyclization of the corresponding vinyl radical onto the O-silyloxime moiety to give cyclic O-silylhydroxylamines in good yields. The reactivity of O-silyloximes in radical cyclization was similar to or even higher than that of O-benzyloximes. Facile removal of the silyl group of the cyclization products leading to hydroxylamines and nitrone formation of the hydroxylamines were also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...