Mechanistic insights into the selective cyclization of indolines with alkynes and alkenes to produce six- and seven-membered 1,7-fused indolines via Rh(iii) catalysis: a theoretical study†
Abstract
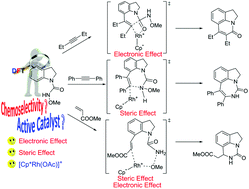
The coupling reaction mechanisms of the Rh(III)-catalyzed redox-neutral C7-selective aryl C–H functionalization of indolines with alkynes and alkenes have been theoretically investigated with the aid of the density functional theory (DFT) calculations. Our calculation results indicate that the active catalyst in this system is the cationic species [Cp*Rh(OAc)]+ (2cat) instead of the neutral species Cp*Rh(OAc)2 (1cat). The origin of forming different products associated with using different coupling partners was also rationalized in detail. For the coupling reaction of N-methoxycarbamoyl-protected indoline (1a) with alkyl alkyne (4a), the electronic effect plays a dominant role and causes the six-membered ring product to be the main product. For the coupling reaction of 1a with aryl alkyne (2a), through the replacement of alkyl alkyne with aryl alkyne, the steric effect serves as a crucial factor, compared with the electronic effect, and leads to the main seven-membered ring product. For the coupling reaction of 1a with acrylate (6a), the chemoselectivity is dictated by the steric effect and electronic effect.


 Please wait while we load your content...
Please wait while we load your content...