β-Amyrin biosynthesis: catalytic mechanism and substrate recognition†
Abstract
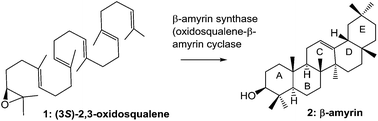
The enzymatic polycyclization reactions catalyzed by oxidosqualene (OXSQ) cyclases (OSCs) proceed with complete regio- and stereospecificity, leading to the formation of new C–C bonds and chiral centers and to the generation of diverse polycyclic sterols and triterpenoids. The diverse structural array is remarkable, and approximately 150 different carbon frameworks have been found. Detailed investigations on squalene-hopene cyclase (SHC) and lanosterol synthase (LaS) have been reported, but progress in the study of β-amyrin synthase, which is ubiquitously found in plants, has lagged in comparison. In the past several years, remarkable advances in β-amyrin biosynthetic studies have been made. In this review, the catalytic mechanism and substrate recognition of β-amyrin synthase, as revealed by site-directed mutagenesis and substrate analog experiments, are outlined and compared with those of LaS and SHC to highlight the features of β-amyrin synthase.
- This article is part of the themed collection: Polycyclic Reactions in Synthesis and Biosynthesis


 Please wait while we load your content...
Please wait while we load your content...