Selective synthesis of mono- and bis-butenolide α-aminomethyl adducts†
Abstract
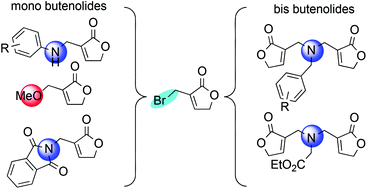
The selective installation of α-methylamine residues at the butenolide core is described using α-bromomethylene-γ-butenolide and primary as well as secondary amines in methanol at 0 °C. The preparation of mono- and bis-butenolide α-adducts is described. Bis-γ-butenolide adducts as well as mono α-aminomethyl-γ-butenolides can be selectively obtained depending on the nature of the reacting primary amine. In contrast, the use of secondary amines allows two different pathways leading either to the expected amino derivatives or to the formation of a C–O bond.


 Please wait while we load your content...
Please wait while we load your content...