Metal-free oxidative ring contraction of benzodiazepinones: an entry to quinoxalinones†
Abstract
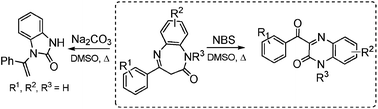
A novel and practical synthesis of 3-benzoylquinoxalin-2(1H)-ones from benzodiazepin-2-ones in two steps from commercially available starting materials is reported. The reaction was achieved in the presence of N-bromosuccinimide in DMSO which served both as a solvent and an oxidant. Significantly, the yet unknown ketone to alcohol fluorescence turn-on of benzoylquinoxalinones was unveiled through the preparation of a fluorescently labelled cholesterol conjugate.


 Please wait while we load your content...
Please wait while we load your content...