A redox-economical synthesis of trifluoromethylated enamides with the Langlois reagent†
Abstract
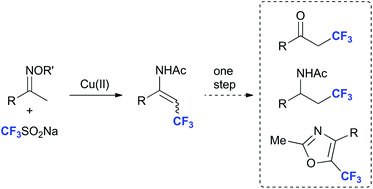
A redox-economical strategy for the synthesis of trifluoromethylated enamides using copper catalysis is reported. The reaction employs the inexpensive Langlois reagent (CF3SO2Na) and takes place without the need of an external oxidant. The trifluoromethylated enamide products can easily be converted into the corresponding ketone, saturated amide or oxazole.


 Please wait while we load your content...
Please wait while we load your content...