Spirooxindole synthesis via palladium-catalyzed dearomative reductive-Heck reaction†
Abstract
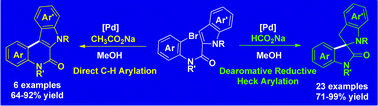
Palladium-catalyzed intramolecular dearomative reductive-Heck reaction of C2-substituted indoles is developed, which provides access to structurally diverse 3,2′-spiropyrrolidine oxindoles. By changing the hydride source to AcONa base, direct C3-arylation products [2,3-b]quinolinones are achieved in good yields. The reaction of C2-substituted benzofuran is also realized, delivering the desired spiro-product.


 Please wait while we load your content...
Please wait while we load your content...