N1-Selective alkenylation of 1-sulfonyl-1,2,3-triazoles with alkynes via gold catalysis†
Abstract
A N1-selective alkenylation of 1-sulfonyl-1,2,3-triazoles with alkynes via gold catalysis is reported. N1-Vinyl substituted 1,2,3-triazoles were selectively prepared in up to 92% yield through the sulfonyl group of 1,2,3-triazole derivatives transformed to alkenyl groups in a “one-pot two steps” manner. This method provided a new method for the synthesis of potentially biological-active vinyl-triazole building blocks.
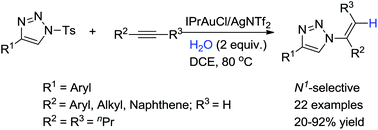


 Please wait while we load your content...
Please wait while we load your content...