Torsional steering as friend and foe: development of a synthetic route to the briarane diterpenoid stereotetrad†
Abstract
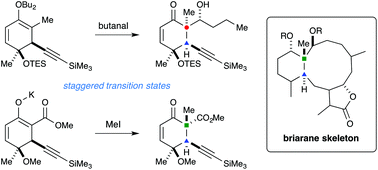
Two synthetic routes to the briarane stereotetrad have been investigated. The first route employed a boron aldol reaction to establish the stereogenic all-carbon quaternary carbon (C1). In this case, it was found that torsional steering in the transition state led to the formation of the undesired configuration at this position. The second route makes use of a highly diastereoselective acetylide conjugate addition/β-ketoester alkylation sequence to construct the vicinal C1 and C10 stereocenters with the correct relative configuration. Originally, it was proposed that torsional steering in the transition state for the ketoester alkylation step was the primary factor responsible for generating the major product. DFT calculations reveal that while torsional steering does play a role, larger conformational factors must also be considered. These calculations also reveal that an unusual C–H⋯π(alkyne) interaction may contribute to lowering the energy of one transition state that leads to the observed stereoisomer. Ultimately, this strategy leads to a concise synthesis (under 10 steps) of the stereotetrad core common to the briarane diterpenoids.


 Please wait while we load your content...
Please wait while we load your content...