Efficient synthesis of polymethoxyselenoflavones via regioselective direct C–H arylation of selenochromones†
Abstract
Substantial research has suggested that the configuration and the total number of functional groups on flavones influence their bioactivity. To investigate the changes in the biological activities of selenoflavones in relationship to structural changes, the development of a generally applicable synthetic method was a key. Until now, an efficient pathway for palladium-catalyzed direct arylation with the selenocyclic enone systems is not known in the literature. We herein introduce a simple direct C–H arylation of two difficult coupling partners, selenochromones and electron-rich aryl bromide, affording diverse polymethoxyselenoflavones with great efficiency and high selectivity.
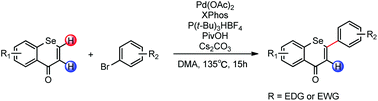


 Please wait while we load your content...
Please wait while we load your content...