Peptide Weinreb amide derivatives as thioester precursors for native chemical ligation†
Abstract
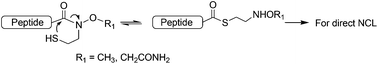
Peptide Weinreb amide derivatives with an N-substituted mercaptoethyl group are designed as thioester precursors for native chemical ligation. We show that these amides undergo rapid ligation with a cysteinyl peptide under normal NCL conditions to form various Xaa–Cys peptide bonds, including the difficult Val–Cys junction. Facile synthesis of the Weinreb amide linkers allows easy access to this new type of peptide thioester precursor by standard Fmoc solid phase synthesis.


 Please wait while we load your content...
Please wait while we load your content...