Syntheses of C17–C27 fragments of 20-deoxybryostatins for assembly using Julia and metathesis reactions†
Abstract
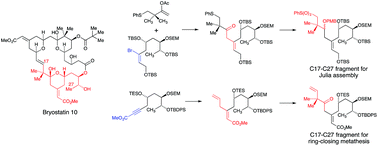
Two approaches to the synthesis of compounds corresponding to the C17–C27 fragment of the 20-deoxybryostatins are described. The first approach is based on the palladium(0) catalysed coupling of tin enolates, generated in situ from enol acetates using tributyltin methoxide, with vinylic bromides. The vinylic bromides were prepared using the Sharpless asymmetric dihydroxylation to introduce the hydroxyl groups corresponding to those at C25 and C26 in the bryostatins. Following several steps to introduce alkynyl ester functionality, the stereoselective addition of a tributyltin cuprate followed by tributyltin–bromine exchange gave the required vinylic bromides. The palladium(0) catalysed couplings worked very well for enol esters containing thioether substituents and gave products with retention of the position and geometry of the trisubstituted double bond derived from the vinylic bromide. These were taken through to compounds corresponding to fully developed C17–C27 fragments ready for assembly of the 16,17-double-bond of bryostatins by Julia reactions. This chemistry was also applied to prepare intermediates suitable for incorporation into bryostatins by ring-closing metathesis but, in this case, the coupling reaction gave mixtures of products including both the required βγ-unsaturated ketone and a conjugated diene formed by a competing Heck reaction. To avoid this problem, a second approach to compounds suitable for incorporation into a metathesis-based assembly of 20-deoxybryostatins was developed. In this organotin-free synthesis, the key step was the conjugate addition of an organic cuprate generated from allylmagnesium bromide to an alkynoate that gave the required (Z)-trisubstituted alkene with excellent stereoselectivity. This was converted into metathesis precursors in a few steps.


 Please wait while we load your content...
Please wait while we load your content...