Discovery and optimization of selective inhibitors of protein arginine methyltransferase 5 by docking-based virtual screening†
Abstract
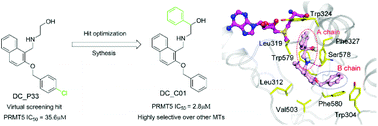
Protein arginine methyltransferase 5 (PRMT5) is a type II PRMT enzyme critical for diverse cellular processes and different types of cancers. Many efforts have been made to discover novel scaffold PRMT5 inhibitors. Herein, we report the discovery of DC_P33 as a hit compound of PRMT5 inhibitor, identified by molecular docking based virtual screening and 3H-labeled radioactive methylation assays. Structure–activity relationship (SAR) analysis was performed on the analogs of DC_P33 and then structural modifications were done to improve its activity. Among the derivatives, the compound DC_C01 displayed an IC50 value of 2.8 μM, and good selectivity toward PRMT1, EZH2 and DNMT3A. Moreover, DC_C01 exhibited anti-proliferation activities against Z-138, Maver-1, and Jeko-1 cancer cells with EC50 values of 12 μM, 12 μM, and 10.5 μM, respectively. Taken together, these results contribute to the development of specific inhibitors against PRMT5 and cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...