A facile and general acid-catalyzed deuteration at methyl groups of N-heteroarylmethanes†
Abstract
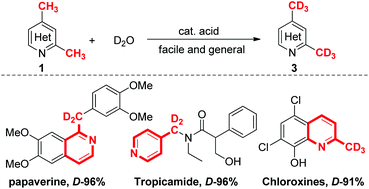
A facile and general Brønsted acid-catalyzed deuteration at the methyl group of N-heteroarylmethanes was achieved through a dearomatic enamine intermediate under relatively mild reaction conditions. Both 2-methyl and 4-methyl groups in quinolines were deuterated with high deuterium incorporation. Pyridines, benzo[d]thiazoles, indoles and imines including these clinical drugs were also deuterated efficiently at the methyl groups. This reaction could be conducted on a large scale (500 mmol), showing its good potential for use in large-scale synthesis.


 Please wait while we load your content...
Please wait while we load your content...