A stereoselective synthesis of (E)- or (Z)-β-arylvinyl halides via a borylative coupling/halodeborylation protocol†
Abstract
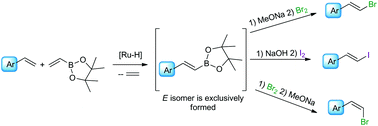
A new stereoselective method for the synthesis of (E)-β-arylvinyl iodides and (E)- or (Z)-β-arylvinyl bromides from styrenes and vinyl boronates on the basis of a one-pot procedure via borylative coupling/halodeborylation is reported. Depending on the halogenating agent as well as the mode of the halodeborylation reaction, (E) or (Z) isomers are selectively formed.


 Please wait while we load your content...
Please wait while we load your content...