Selective S-arylation of 2-oxazolidinethiones and selective N-arylation of 2-benzoxazolinones/2-benzimidazolinones†
Abstract
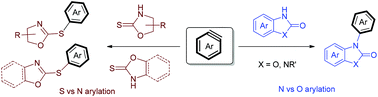
There exist three possible patterns for the reaction of cyclic 2-oxazolidinethione and 2-benzoxazolidinethione with arynes, namely (a) S-arylation, (b) N-arylation, and (c) aryne insertion into the thiocarbonyl group (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S). Our studies demonstrate that S-arylation wins out affording S-aryl dihydrooxazoles. In contrast, for related reactions of cyclic 2-benzoxazolinone and 2-benzimidazolinone with arynes, it is found that N-arylation outcompetes O-arylation and aryne insertion into the C
S). Our studies demonstrate that S-arylation wins out affording S-aryl dihydrooxazoles. In contrast, for related reactions of cyclic 2-benzoxazolinone and 2-benzimidazolinone with arynes, it is found that N-arylation outcompetes O-arylation and aryne insertion into the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group to give N-aryl 2-benzoxazolinones and N-aryl 2-benzimidazolinones.
O group to give N-aryl 2-benzoxazolinones and N-aryl 2-benzimidazolinones.


 Please wait while we load your content...
Please wait while we load your content...