One-pot synthesis of α,β-epoxy ketones through domino reaction between alkenes and aldehydes catalyzed by proline based chiral organocatalysts†
Abstract
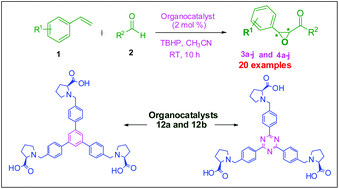
Proline based metal free organocatalysts were developed by using a new approach for the synthesis of epoxide derivatives through a domino reaction. This domino reaction (oxidative coupling) allows a direct access to epoxides from various alkenes and aldehydes through C–H functionalization and C–C/C–O bond formation. The catalytic efficiencies of the newly synthesized organocatalysts were also determined by domino reaction in the presence of various functional groups containing aldehyde and alkene derivatives with very good yields (up to 95%) and ee's (up to 99%).


 Please wait while we load your content...
Please wait while we load your content...