Enzymatic cascades for the stereo-complementary epimerisation of in situ generated epoxy alcohols†
Abstract
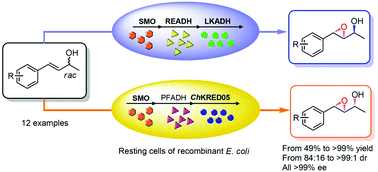
The synthesis of optically pure secondary epoxy alcohols from racemic allylic alcohols using a single whole-cell biocatalyst of recombinant Escherichia coli coexpressing three oxidoreductases is described. The cascade involves the concurrent action of a styrene monooxygenase that catalyzes the formation of the chiral epoxy group, and two alcohol dehydrogenases that fulfil the epimerisation of the hydroxy group. Two sets of alcohol dehydrogenases were each applied to couple with styrene monooxygenase in order to realize the epimerisation in a stereo-complementary manner. Excellent enantio- and diastereo-selectivities were achieved for most of the 12 substrates.


 Please wait while we load your content...
Please wait while we load your content...