Synthesis, radio-synthesis and in vitro evaluation of terminally fluorinated derivatives of HU-210 and HU-211 as novel candidate PET tracers†
Abstract
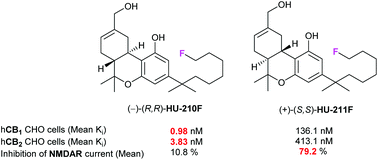
We report the synthesis of terminally fluorinated HU-210 and HU-211 analogues (HU-210F and HU-211F, respectively) and their biological evaluation as ligands of cannabinoid receptors (CB1 and CB2) and N-methyl D-aspartate receptor (NMDAR). [18F]-labelled HU-210F was radiosynthesised from the bromo-substituted precursor. In vitro assays showed that both HU-210F and HU-211F retain the potent pharmacological profile of HU-210 and HU-211, suggesting that [18F]-radiolabelled HU-210F and HU-211F could have potential as PET tracers for in vivo imaging.


 Please wait while we load your content...
Please wait while we load your content...