Structure–activity relationships of the ATP cofactor in ligase-catalysed oligonucleotide polymerisations†
Abstract
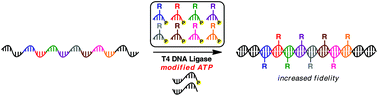
A T4 DNA ligase-catalysed oligonucleotide polymerisation process has been recently developed to enable the incorporation of multiple functional groups throughout a nucleic acid polymer. T4 DNA ligase requires ATP as a cofactor to catalyse phosphodiester bond formation during the polymerisation process. Herein, we describe the structure–activity relationship of ATP within the context of T4 DNA ligase-catalyzed oligonucleotide polymerisation. Using high-throughput sequencing, we study not only the influence of ATP modification on polymerisation efficiency, but also on the fidelity and sequence bias of the polymerisation process.


 Please wait while we load your content...
Please wait while we load your content...