Betti's base for crystallization-induced deracemization of substituted aldehydes: synthesis of enantiopure amorolfine and fenpropimorph†
Abstract
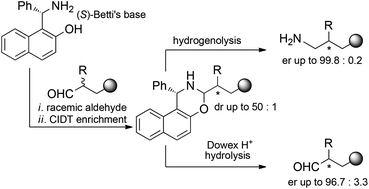
The acid-promoted crystallization-induced diastereoisomer transformation (CIDT) of naphthoxazines derived from racemic O-protected 2-substituted 4-hydroxybutyraldehydes and enantiopure Betti's base allows the deracemization of the starting aldehydes with ee up to 96%. As an alternative, reduction with lithium aluminum hydride of the diastereoisomerically enriched naphthoxazines leads to enantioenriched primary amines. The utility of the latter strategy was demonstrated by applying it to the synthesis of enantioenriched fenpropimorph and to the first synthesis of enantiopure amorolfine, with ee up to 99.5%.


 Please wait while we load your content...
Please wait while we load your content...